Beyond Antibiotics
Beyond Antibiotics: A Programme Grant funded by the Engineering and Physical Sciences Research Council (EPSRC)
The 2019 World Health Organisation (WHO) report on Antimicrobial Resistance (AMR) identifies it as: “one of the greatest threats we face as a global community.” The evolution of drug-resistant bacteria, our over-use of antibiotics and failure to develop new methods for tackling infection could leave us without viable treatments for even the most trivial infections within the next 3 decades.
There have been significant efforts by the WHO and national agencies to raise awareness of AMR and reduce the use of antibiotics, but there is still an urgent need to intensify these efforts and, crucially, to develop alternatives. The aim of the programme is to address this need.
The programme consists of 4 interlinked work packages (WPs) focussed on the core research objectives:
(1) The development of human organoid models for studying interactions between bacteria and the tissue microenvironment and larger scale interactions with the host microbiome.
(2) New microscopy methods to complement the organoid models and to facilitate rapid characterisation of bacteria for improved diagnosis.
(3) New antimicrobial therapeutics and targeted delivery techniques to improve the use of existing antibiotics and provide viable alternatives.
(4) “Drug-free” methods for treating infections and promoting immune function thereby further reducing the use of antibiotics and providing methods suitable for large scale environmental/industrial use.
Funder
UKRI - Engineering and Physical Sciences Research Council (EPSRC). Programme Grant Scheme EP/V026623/1.
Overview of Research

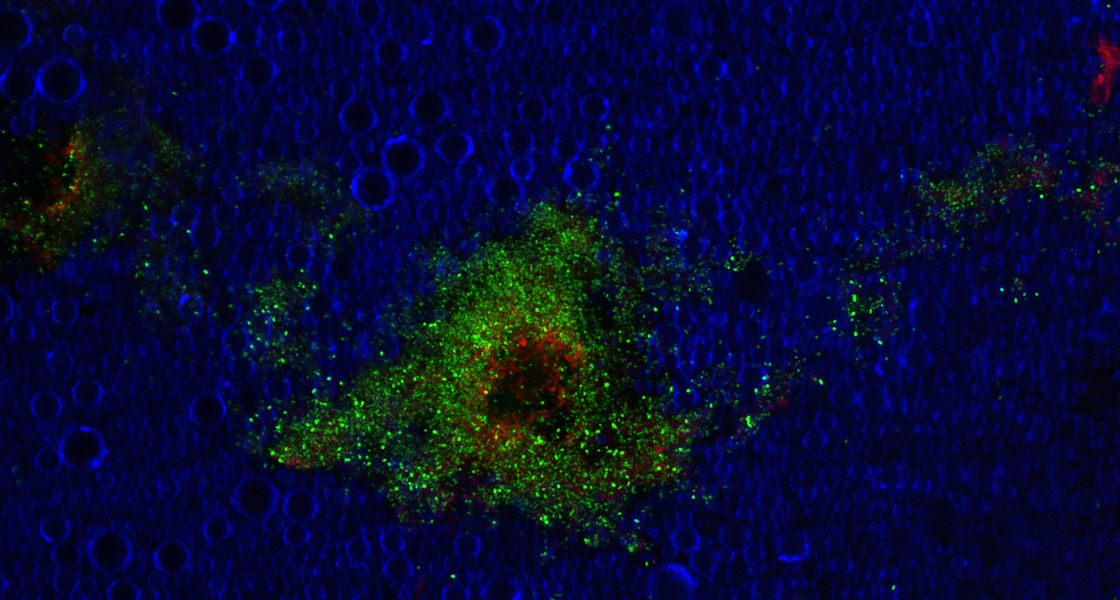
Work Package 1 focuses on the creation of new platforms for investigating the interactions between bacteria, their microenvironment and therapeutic interventions. Organoid models of the bladder and gut have been established. Biofilms have been grown onto organoid models grown statically and have been evaluated through confocal microscopy and colony-forming unit counting.
Images (left) show microscopic characterisation of a bladder organoid, co-infected with two pathogens.
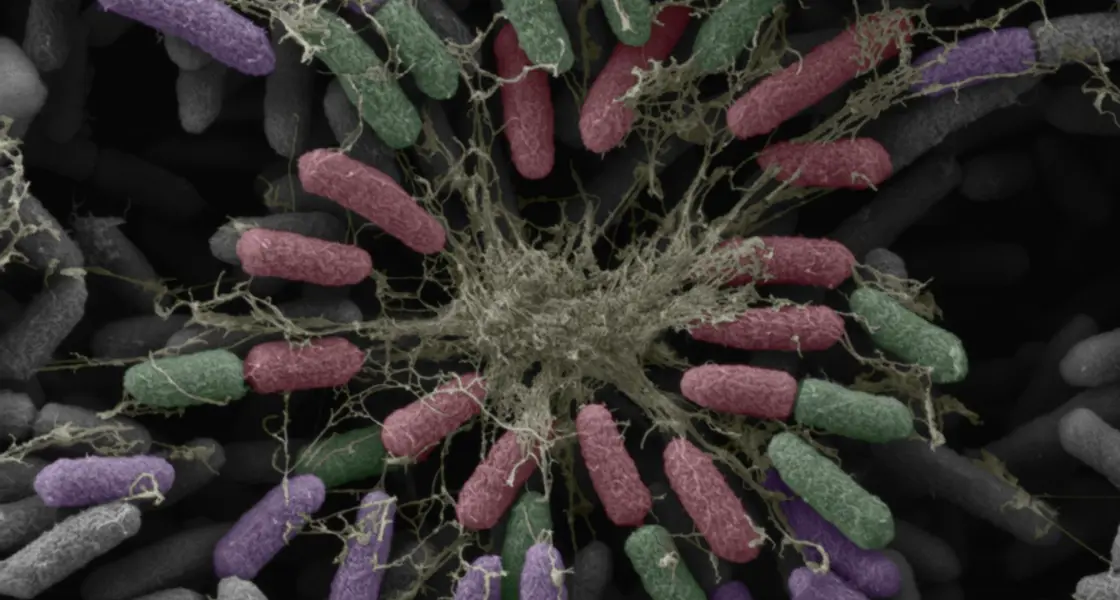
Work Package 2 focuses on developing microscopy methods for the rapid identification of resistant bacterial strains as an indicator of treatment efficacy. The combination of confocal fluorescence with high-resolution electron microscopy has enabled the three-dimensional structural characterisation of biofilms. Spectroscopic methods have been used to assess biofilm integrity and dispersal.
Image (left) shows bacteria and extracellular matrix fibres forming a biofilm that hinders antibiotic drug delivery.
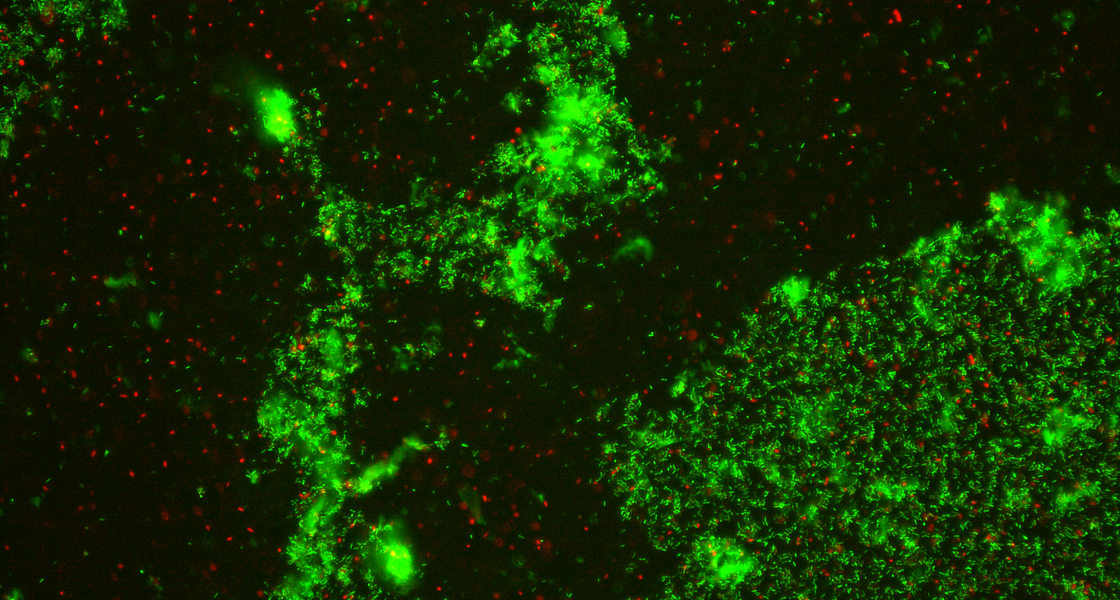
Work Package 3 investigates methods for improving the delivery of existing and new antimicrobial drugs, including biologic agents to inhibit the development of resistance. Through micro-and nanoencapsulation, new formulations that allow for targeted or stimuli-responsive delivery are being developed.
Image (left) shows a cluster of bacteria after exposure to perfluorocarbon droplets.
Work Package 4 uses ultra-high-speed imaging and acoustic monitoring to optimise exposure conditions and identify suitable treatment monitoring variables to eliminate bacteria and biofilms through physical treatment approaches, such as ultrasound and shockwave exposure. The impact on immune function of physical stimuli is being assessed to determine toxicity and quantify the potential therapeutic value. Hyperspectral imaging is used in combination with ultrasound exposure to monitor biofilm disruption.
Image (left) shows bacterial biofilm following exposure to protein cavitation nuclei.
Research Impacts to Date

NEW: Research published
"Repurposing antimicrobials with ultrasound-triggered nanoscale systems for targeted biofilm drug delivery"
This research is funded by the Engineering and Physical Science Research Council (EPSRC) Programme Grant Scheme under the reference number EP/V026623/1.









